Advertisement
Latest Version (Download)Table of Contents
Advertisement
Information
| Package | com.electrochemistry.books |
| Version | 20.0.0 |
| Date Updated | 2020-09-26 |
| Size | 6.28 MB |
| Installs | 24 |
| Categories | , |
Screenshots
Description
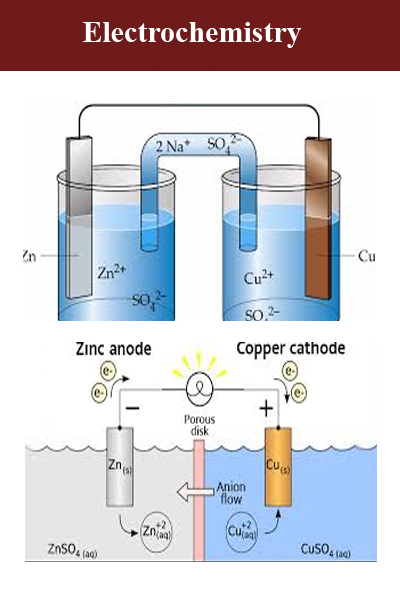
Electrochemistry is the branch of physical chemistry that studies the relationship between electricity, as a measurable and quantitative phenomenon, and identifiable chemical change, with either electricity considered an outcome of a particular chemical change or vice-versa. These reactions involve electric charges moving between electrodes and an electrolyte (or ionic species in a solution). Thus electrochemistry deals with the interaction between electrical energy and chemical change.
When a chemical reaction is caused by an externally supplied current, as in electrolysis, or if an electric current is produced by a spontaneous chemical reaction as in a battery, it is called an electrochemical reaction. Chemical reactions where electrons are transferred directly between molecules and/or atoms are called oxidation-reduction or (redox) reactions. In general, electrochemistry describes the overall reactions when individual redox reactions are separate but connected by an external electric circuit and an intervening electrolyte.
Support Language:
✔العربية
✔català
✔Čeština
✔Deutsch
✔English
✔Español
✔فارْسِى
✔français
✔Magyar
✔հայերեն
✔italiano
✔日本語
✔한국어
✔Nederlands
✔polski
✔Português
✔українська
✔中文
What's New
4.0.0
Latest Version (Download)
Electrochemistry Books 20.0.0Date Updated : 2020-09-26
Advertisement